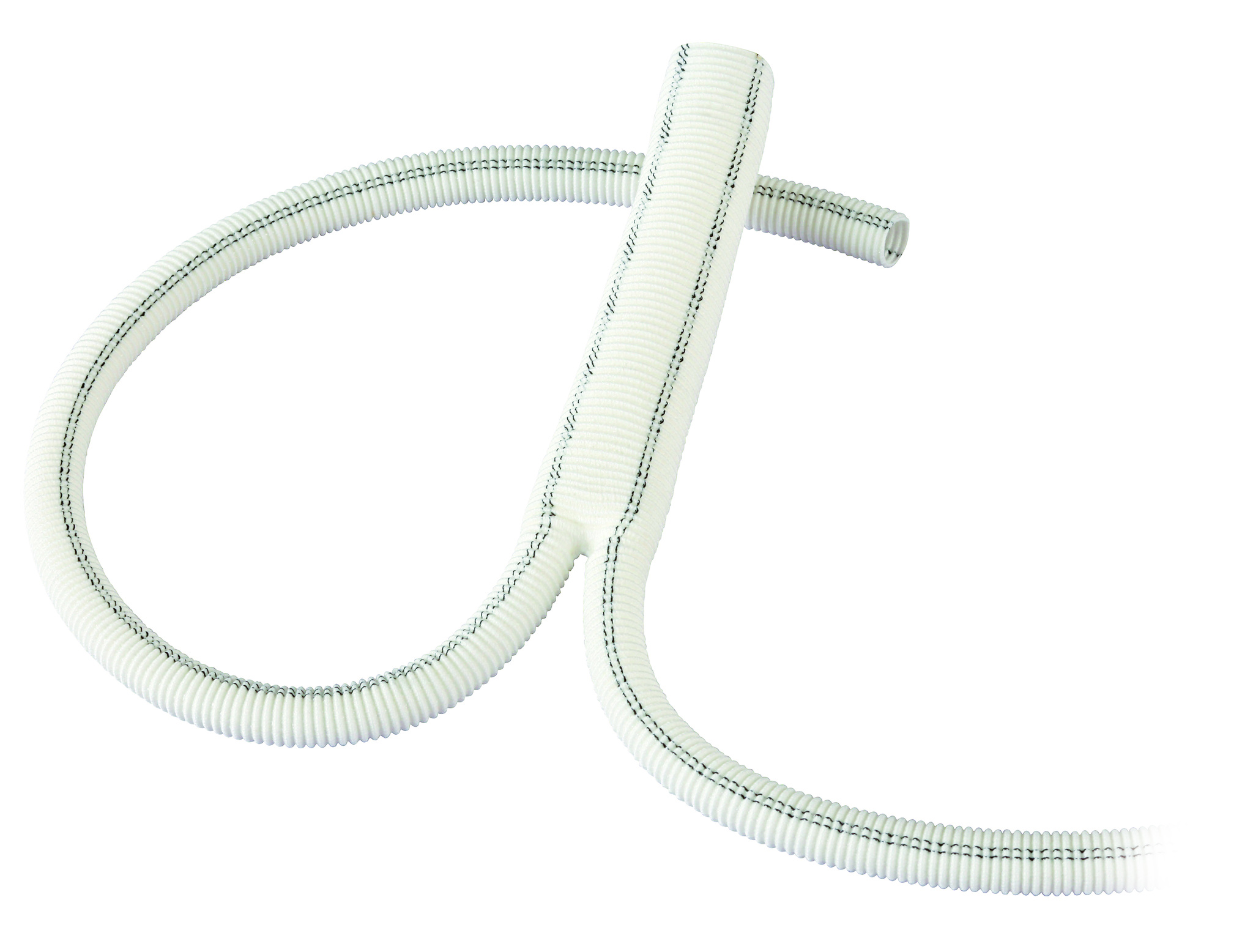
Polythese Woven Dacron
Polythese® IC
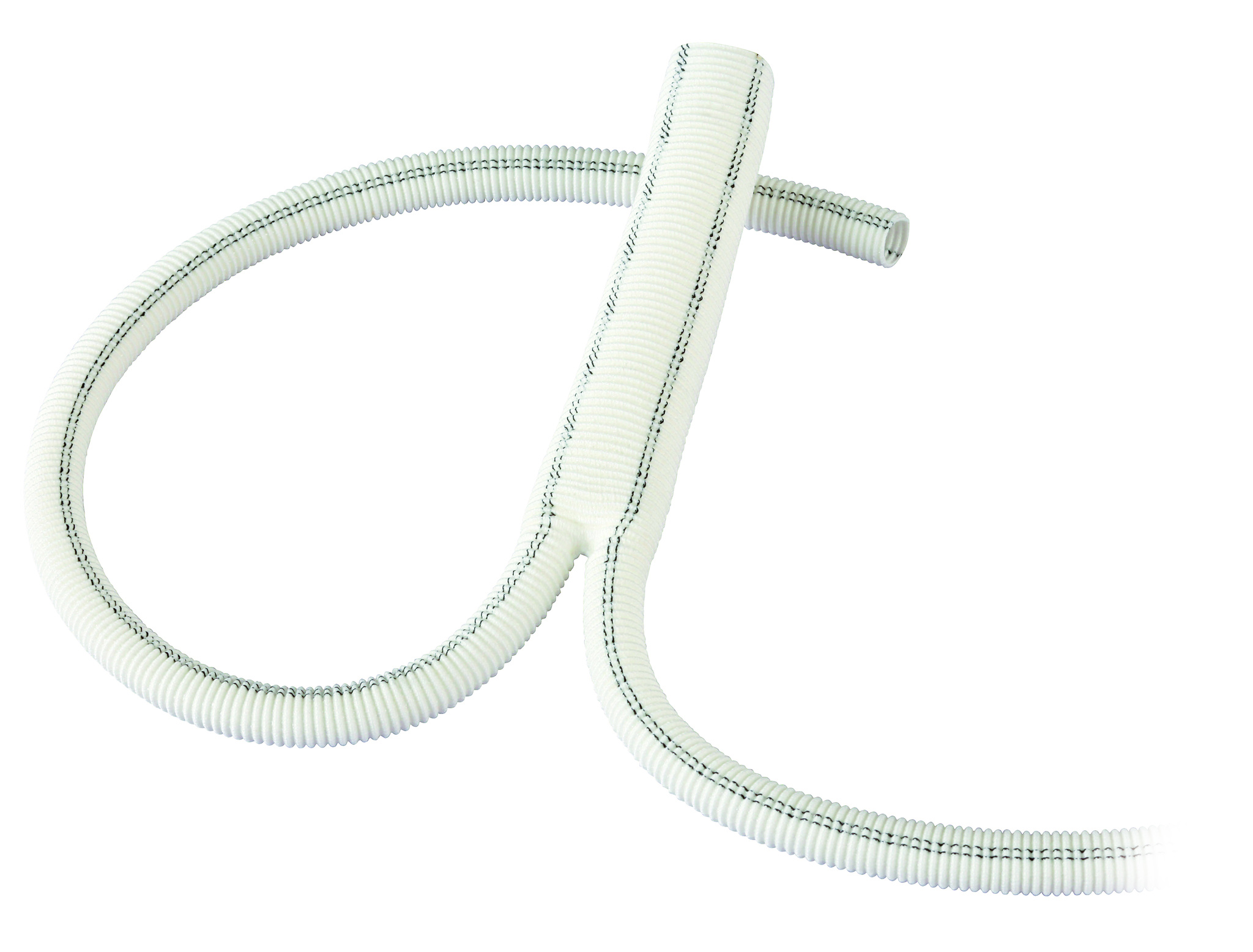
Woven Polyester Vascular Graft
- Wide range of woven grafts designed for thoracic (polythese® ICT), abdominal and peripheral surgery.
- Good suturability and handling.
Features & benefits:
Dimensional stability
- Good resistance to circumferential dilatation (1)
Patented technology of impregnation (collagen cxe®)
- Formaldehyde and Glutaraldehyde free (2)
External velvet-like surface: support for neighbour tissues attachment and periprosthetic encapsulation
Smooth and texturized endoluminal wall: support for pseudo-intimal cells ingrowth
(1) Rapport RRD-0101-01 rev00 - 29/10/2012.
(2) LNE certificate n°F014288 (2005): analysis of Perouse Grafts: formaldehyde free and glutaraldehyde free.
- Contains Latex: No
- Contains DEHP: No
- Contains biogical or animal-based product: Yes
- Non-pyrogenic: Yes
Polythese® ICT
Woven Polyester Vascular Graft
- Wide range of woven grafts designed for thoracic (polythese® ICT), abdominal and peripheral surgery.
- Good suturability and handling.
Features & benefits:
Dimensional stability
- Good resistance to circumferential dilatation (1)
Patented technology of impregnation (collagen cxe®)
- Formaldehyde and Glutaraldehyde free (2)
External velvet-like surface
- Support for neighbour tissues attachment and periprosthetic encapsulation
Smooth and texturized endoluminal wall
- Support for pseudo-intimal cells ingrowth
(1) Rapport RRD-0101-01 rev00 - 29/10/2012.
(2) LNE certificate n°F014288 (2005): analysis of Perouse Grafts: formaldehyde free and glutaraldehyde free.
__________________________________
Additional Information
- Contains Latex: No
- Contains DEHP: No
- Contains biogical or animal-based product: Yes
- Non-pyrogenic: Yes
Polythese® ICT

- Woven Polyester Vascular Graft
- Wide range of woven grafts designed for thoracic (polythese® ICT), abdominal and peripheral surgery.
- Good suturability and handling.
Features & benefits:Dimensional stability
- Good resistance to circumferential dilatation (1)
- Formaldehyde and Glutaraldehyde free (2)
External velvet-like surface
- Support for neighbour tissues attachment and periprosthetic encapsulation
Smooth and texturized endoluminal wall
- Support for pseudo-intimal cells ingrowth
(1) Rapport RRD-0101-01 rev00 - 29/10/2012.
(2) LNE certificate n°F014288 (2005): analysis of Perouse Grafts: formaldehyde free and glutaraldehyde free.
Additional Information
- Contains Latex: No
- Contains DEHP: No
- Contains biogical or animal-based product: Yes
- Non-pyrogenic: Yes